VTX2735 is a novel, highly potent, and selective oral inhibitor of NLRP3 with potential therapeutic benefit in a range of systemic inflammatory conditions including cardiovascular, metabolic, rheumatic, dermatologic, and rare genetic diseases. Initial clinical proof-of-concept for VTX2735 was established in a Phase 2 trial in patients with cryopyrin-associated periodic syndromes (CAPS), a genetic disease involving overactive NLRP3 (due to a naturally occurring gain-of-function mutation in the NLRP3 gene). Treatment with VTX2735 led to meaningful improvement in both inflammatory biomarkers and patient-reported symptom scores in this rare disease indication. These clinical data provide a strong therapeutic rationale for other diseases in which NLRP3 is aberrantly activated.
NLRP3 inhibition is emerging as a compelling therapeutic approach for multiple cardiovascular conditions, offering the potential to selectively modulate IL-1β and induce downstream effects on other inflammatory markers such as IL-6 and CRP with a safe, convenient oral therapy. Ventyx has selected recurrent pericarditis as the initial cardiovascular indication for VTX2735.
Recurrent pericarditis is a rare, debilitating autoinflammatory condition affecting approximately 40,000 patients in the US alone. The inflammatory process in RP is characterized by IL-1α/β release (indirectly and directly downstream of NLRP3, respectively), driving inflammation in the pericardium, the fluid-filled sac surrounding the heart. Rilonacept, an injectable IL-1α/β cytokine trap, became the first FDA-approved therapy for RP in 2021, validating the anti-IL-1α/β approach in RP. A peripheral NLRP3 inhibitor such as VTX2735 may be an effective therapy for the treatment and prevention of recurrent pericarditis, offering a safe, oral option for patients living with RP.
VTX3232 is currently in Phase 2 trials for the treatment of Parkinson’s disease and obesity-associated cardiometabolic diseases.
VTX3232 is an oral, selective, CNS-penetrant NLRP3 inhibitor with potential therapeutic utility for a range of neuroinflammatory diseases, including Parkinson’s disease (PD), Alzheimer’s disease (AD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and cardiometabolic diseases.
First-generation NLRP3 inhibitors have poor inherent blood-brain barrier permeability and require high systemic doses to achieve CNS efficacy.
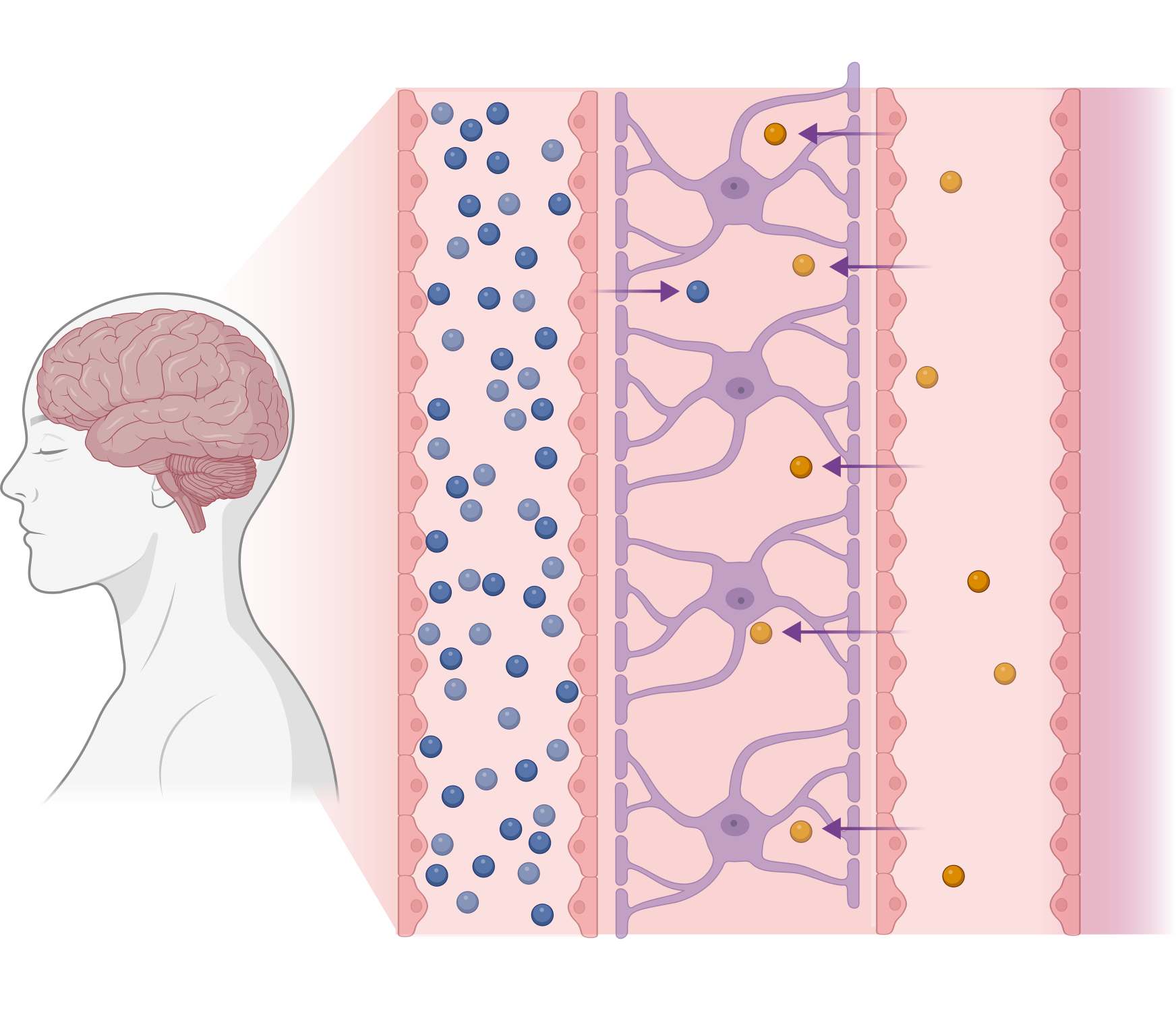
VTX3232 is rationally designed for rapid equilibration across the BBB, efficiently reaching target cells in the brain.
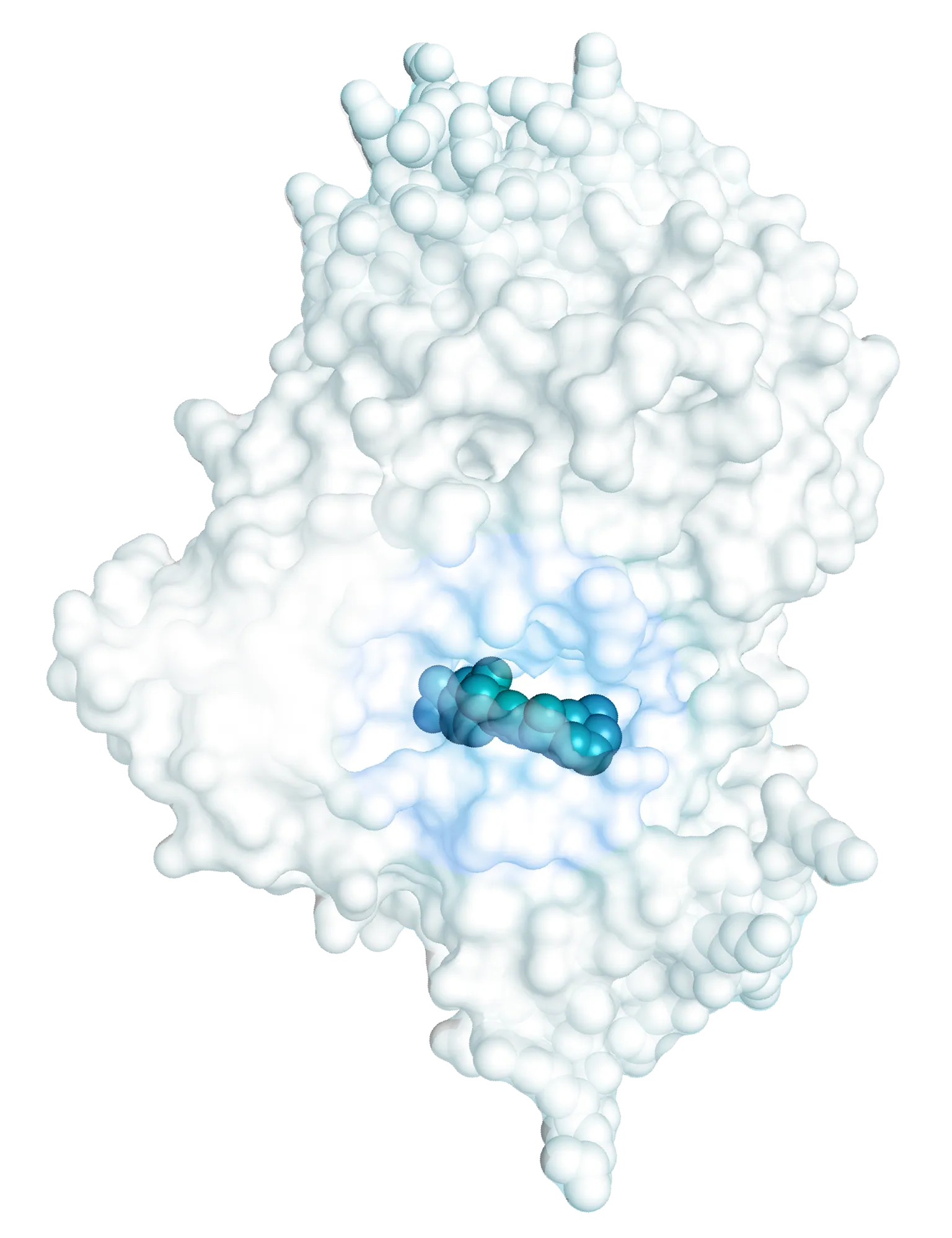
There is a growing body of preclinical evidence implicating microglial NLRP3 activation as an important axis in PD pathogenesis. Preclinical models and PD patient samples strongly implicate NLRP3-mediated neuroinflammation as a key driver of neuronal degeneration in PD. Inhibition of NLRP3 in the CNS may provide a disease-modifying therapeutic approach with potential to prevent dopaminergic neurodegeneration and clinical progression in PD.
NLRP3-mediated inflammation has been implicated in obesity and metabolic disorders, including insulin resistance, diabetes, and atherosclerosis, which are all potentially linked to NLRP3 -derived cytokines such as IL-1β and IL-6 in the periphery. NLRP3-mediated neuroinflammation in the hypothalamus has also been proposed as playing a significant role in obesity and obesity-related cardiometabolic dysfunction. Reducing NLRP3-mediated chronic inflammation, both systemically and in the CNS, with VTX3232 could be an important new method for treating these conditions.
Tamuzimod is a selective S1PR1 modulator that, in a 52-week Phase 2 trial, demonstrated a potential best-in-class profile in UC, with a potential best-in-disease safety profile amongst all the oral options for UC therapy. Tamuzimod showed high rates of clinical remission and endoscopic remission which may position this drug as the backbone of future advanced combination treatment for UC, offering an effective and well-tolerated alternative for achieving optimal disease control in selected patients.
TYK2 regulates both innate and adaptive immunity by mediating type I interferon, IL-12, and IL-23 signaling. Selectively targeting inhibition of TYK2 without inhibition of other JAK family enzymes provides an optimal balance between reducing inflammation and preserving immune protection.
VTX958 has been evaluated in Phase 2 trials in psoriasis and Crohn’s disease. Further development of VTX958 in IBD is currently being evaluated.
Sometimes called “compassionate use”, expanded access is a potential pathway for a patient with a serious or life-threatening disease or condition to try an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when there are no comparable or satisfactory therapies available. Expanded access may be appropriate when all the following apply:
At this time, Ventyx Biosciences, Inc., and its affiliates (referred to as Ventyx), are unable to provide their investigational medicines on an expanded access or right to try basis.
To determine if you qualify for a clinical trial of a Ventyx investigational product, please visit Clinicaltrials.gov.