Inflammasomes are multi-protein complexes that assemble in response to molecular hallmarks of infection or cellular injury and initiate an appropriate innate immune response. NLRP3, the most prominent inflammasome, is activated by signals associated with chronic inflammation and/or tissue damage in a broad range of systemic (cardiometabolic, dermatologic, rheumatic) and neurodegenerative diseases.1 Activated NLRP3 stimulates the production of the inflammatory cytokines IL-1β and IL-18, as well as a lytic type of cell death called pyroptosis, which can lead to further inflammation (i.e. an autoinflammatory feed-forward loop). IL-1β production can stimulate the production of additional inflammatory proteins including IL-6 and, downstream, hsCRP, which is a well-validated marker of inflammation and cardiovascular risk. NLRP3 inhibition has the potential to break the autoinflammatory feed-forward loop that underlies a range of chronic inflammatory diseases and conditions, leading to their resolution.
Learn about our clinical-stage
The role of IL-1β signaling downstream of inflammasome activation has been validated in a broad range of chronic inflammatory disorders, particularly in diseases where anti-IL-1 biologics have demonstrated therapeutic benefit.2 These include periodic fever syndromes, gout, rheumatoid arthritis, hidradenitis suppurativa, and cardiovascular risk reduction, among others. There is also emerging evidence supporting NLRP3 inhibition as a promising therapeutic approach in obesity and cardiometabolic diseases, as well as in a range of debilitating neurodegenerative diseases where NLRP3-mediated neuroinflammation may be deleterious, including Parkinson’s disease, Alzheimer’s disease, and amyotrophic lateral sclerosis (ALS).
1. Swanson et al, Nat Rev Immunol, 2019, 19, 477; Ma, Pharmacol Rev, 2023, 75, 487
2. Sims et al, Nat Rev Immunol, 2010, 10, 89; Mantovani et al, Immunity, 2019, 50(4), 778
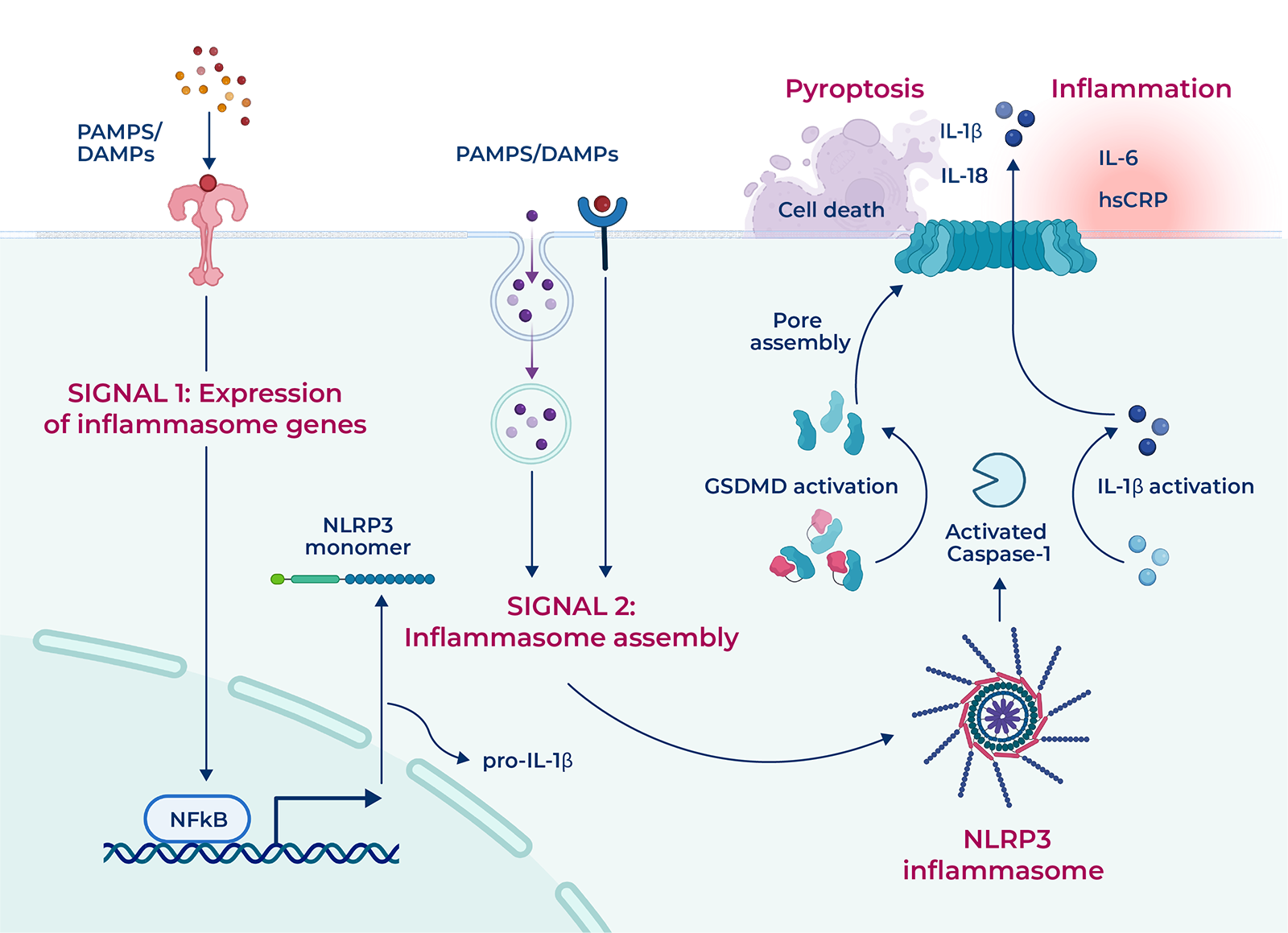
NLRP3 activation, initiated upon sensing of cellular damage signals, involves two critical stages: inflammasome priming and assembly. The assembled NLRP3 complex initiates the activation and release of potent inflammatory cytokines IL-1β and IL-18 as well as the cellular death process known as pyroptosis.2 Preclinical and clinical data associate NLRP3 activation with over 20 indications characterized by aberrant inflammation.

Chronic inflammation in the brain is increasingly understood as a major contributor to the progressive neuronal loss that defines neurodegenerative diseases, and a vast amount of data support NLRP3 as a potential trigger for the inflammatory process. NLRP3 is activated in the CNS by stimuli including but not limited to acute inflammatory trauma as in traumatic brain injury (TBI) and stroke, by an autoimmune-mediated trigger in multiple sclerosis (MS), and in response to an accumulation of misfolded or aggregated proteins in the brain of Alzheimer’s (amyloid-β) and Parkinson’s patients (α-synuclein).1 Parkinson’s disease (PD) is the second most common neurodegenerative disease in the US,2 affecting approximately 1 million people, with currently no approved disease-modifying therapies available. Evidence is growing that the misfolded α-synuclein protein, the accumulation of which is one of the defining pathological features of PD, directly activates the NLRP3 inflammasome in microglia, the immune cells of the brain, to drive neuroinflammation and ultimately neuronal damage and degeneration.3,4
PD patients have upregulated NLRP3 expression in the brain and higher levels of NLRP3-derived cytokines (e.g. IL-1β, IL-18).5 In multiple animal models of PD, NLRP3 inhibition reduces motor symptom severity, inflammatory microglial activation, and dopaminergic neuron loss.4-6 NLRP3 inhibition represents a potential novel mechanism for disease modification in Parkinson’s disease that may preserve neuronal function and prevent the onset or progression of clinical symptoms associated with the disease.
1. Voet S et al, EMBO Mol Med, 2019, 11(6), e10248
2. Ben-Shlomo et al, The Lancet, 2024, 403(10423), 283
3. Pike et al, Glia, 2021, 69(6), 1413; Huang et al, Biomedicine & Pharmacotherapy, 2023, 168, 115735
4. Gordon et al, Sci Trans Med, 2018, 10(465), eaah4066
5. Von Herrmann et al, NPJ Parkinson’s Dis, 2018, 15(4), 24; Anderson et al, NPJ Parkinson’s Dis, 2021, 7, 2; Panicker et al, Neuron, 2022, 110(15), 2422
6. Huang et al, J Neuroimmunol, 2021, 354, 577543; Grotemeyer et al J Neuroinflammation, 2023, 20(1), 79; Chatterjee et al, Brain Research, 2024, 1842, 149129
Recurrent pericarditis (RP) is a rare, debilitating, autoinflammatory condition affecting approximately 40,000 patients in the US alone.1 The inflammatory process in RP is characterized by IL-1 release downstream of NLRP3,2 driving inflammation in the pericardium, the fluid-filled sac surrounding the heart. Patients experience sharp chest pains and often have difficulty breathing. Rilonacept, an injectable IL-1α/β cytokine trap, became the first FDA-approved therapy for RP in 2021, validating the IL-1 pathway as a key driver of symptomatic disease.3 NLRP3 inhibitors may be an effective next-generation therapy for the treatment and prevention of recurrent pericarditis, offering a safe, oral option for patients living with RP.
1. Klein et al, J Am Heart Assoc, 2021, 10, e018950
2. Mauro et al, JACC: Basic to Translational Science, 2021, 6(2), 137; Toldo et al, Nat Rev Cardiol, 2024, 21, 219
3. Klein et al, N Engl J Med, 2021, 384, 31
Disorders affecting the heart and blood vasculature are generally referred to as cardiovascular (CV) diseases, and include atherosclerosis, acute myocardial infarction, heart failure, pericarditis, and others. These diseases have a recognized inflammatory component, and activation of the NLRP3 inflammasome with the resultant production of IL-1β and IL-18 has been directly linked to the pathogenesis of these diseases and conditions.1 Late-stage clinical trials targeting cytokines that are released downstream of NLRP3 activation have demonstrated clear therapeutic benefit to patients with CV disease, including:
Canakinumab, ziltivekimab, and other cytokine-targeted therapies are injectable macromolecules. Small molecule NLRP3 inhibitors provide an effective, safe, and convenient oral option for reducing the risk of CV disease across this large patient population by acting upstream of IL-1β and IL-18 production.
1. Takahashi, Cardiovascular Research, 2022, 118(2), 372; Zheng et al, Front Cardiovasc Med, 2022, 9, 927061
2. Ridker et al, N Engl J Med, 2017, 377, 1119
3. Ridker et al, The Lancet, 2021, 397(10289), 2060; Ridker et al, Cardiovascular Research, 2021, 117(11), e138
Cardiometabolic disease refers to the complex set of pathologies, symptoms, and elevated risk factors that are all driven by an underlying perturbation in the homeostasis of energy signaling and body composition. Processes that become dysregulated include insulin signaling, lipid metabolism, energy utilization, and inflammation, and affect multiple organs including the brain, liver, gastrointestinal system, and adipose tissue with profound clinical implications for cardiovascular disease, diabetes, metabolic dysfunction-associated steatohepatitis (MASH), and chronic kidney disease (CKD).1
Inflammasomes have emerged as a critical link between metabolism and inflammation, and activation of the NLRP3 inflammasome and subsequent release of pro-inflammatory cytokines IL-1β and IL-18 has been well documented in the pathogenesis of metabolic diseases.2 For example, NLRP3 can be activated by cholesterol crystals in atherosclerosis,3 uric acid crystals in gout,4 and saturated fatty acids in inflammatory liver diseases like metabolic dysfunction-associated steatotic liver disease (MASLD) and MASH.5 NLRP3 inhibitors are a promising new approach to addressing the chronic inflammation underlying cardiometabolic diseases, which affect 20-25% of the general population.6
1. Chavakis et al, Cardiovascular Research, 2023, 119(18), 2771
2. De Nardo et al, Trends Immunol, 2011, 32(8), 373; Haneklaus et al, Immunol Rev, 2015, 265(1), 53
3. Karasawa et al, J Atheroscler Thromb, 2017, 24(5), 443
4. Kim, J Rheum Dis, 2022, 29(3), 140
5. Dong et al, Ann Transl Med, 2020, 8(5), 168; Yu et al, Front Pharmacol, 2022, 13, 780496
6. Khan et al, Cureus, 2023, 15(9), e45542
Obesity occurs when energy homeostasis is disrupted.1 Under normal conditions, adipose tissue closely communicates with the brain to maintain energy homeostasis and body weight by secreting a variety of humoral factors, called adipokines. The brain, particularly the hypothalamus, senses and integrates these signals and maintains energy balance by controlling feeding behavior and energy expenditure.2 Chronic low-grade inflammation is observed in subjects with obesity, both peripherally in adipose tissues and liver and centrally in the hypothalamus.2,3
In mice, NLRP3 deficiency has been broadly demonstrated as protective against diet-induced obesity and associated pathologies.4 Furthermore, our internal preclinical studies have demonstrated that VTX3232 lowers body weight and body weight gain in diet-induced obese mice with associated reduction in obesity-associated inflammatory markers both in the peripheral circulation and in the brain.
1. Hill et al, Circulation, 2012, 126(1), 126
2. Marcos et al, Int J Mol Sci, 2023, 24(2), 1468
3. De Souza et al, Endocrinology, 2005, 146(10), 4192; Johnson et al, Cell, 2013, 152(4), 673; Weisberg et al, J Clin Invest, 2003, 112(12), 1796; Thaler et al, 2012, J Clin Invest, 122(1), 153
4. Stienstra et al, Proc Natl Acad Sci USA, 2011, 108, 15324; Vandanmagsar et al, Nat Med, 2011, 17, 179; Wen et al, Nat Immunol, 2011, 12, 408; Sokolova et al, Front Immunol, 2019, 10, 1621; Sokolova et al, Sci Rep, 2020, 10(1), 21006; Pellegrini et al, Br J Pharmacol, 2021, 178(19), 3924
S1P receptors are G-protein–coupled receptors that control lymphocyte trafficking from lymph nodes to the peripheral blood.1 Modulators of sphingosine-1-phosphate receptor 1 (S1P1R) sequester lymphocytes in the lymph nodes. The resulting decrease in lymphocyte egress leads to fewer immune cells reaching the site of inflammation in diseases such as ulcerative colitis, promoting tissue healing and symptom resolution.
1. O’Sullivan et al, Trends Pharm Sci, 2013, 34(7), 401; Bravo et al, Cells, 2022, 11(13), 2058
Learn about our program
Lymphocyte migration from lymphoid organs to the site of inflammation is controlled by the signaling molecule S1P.
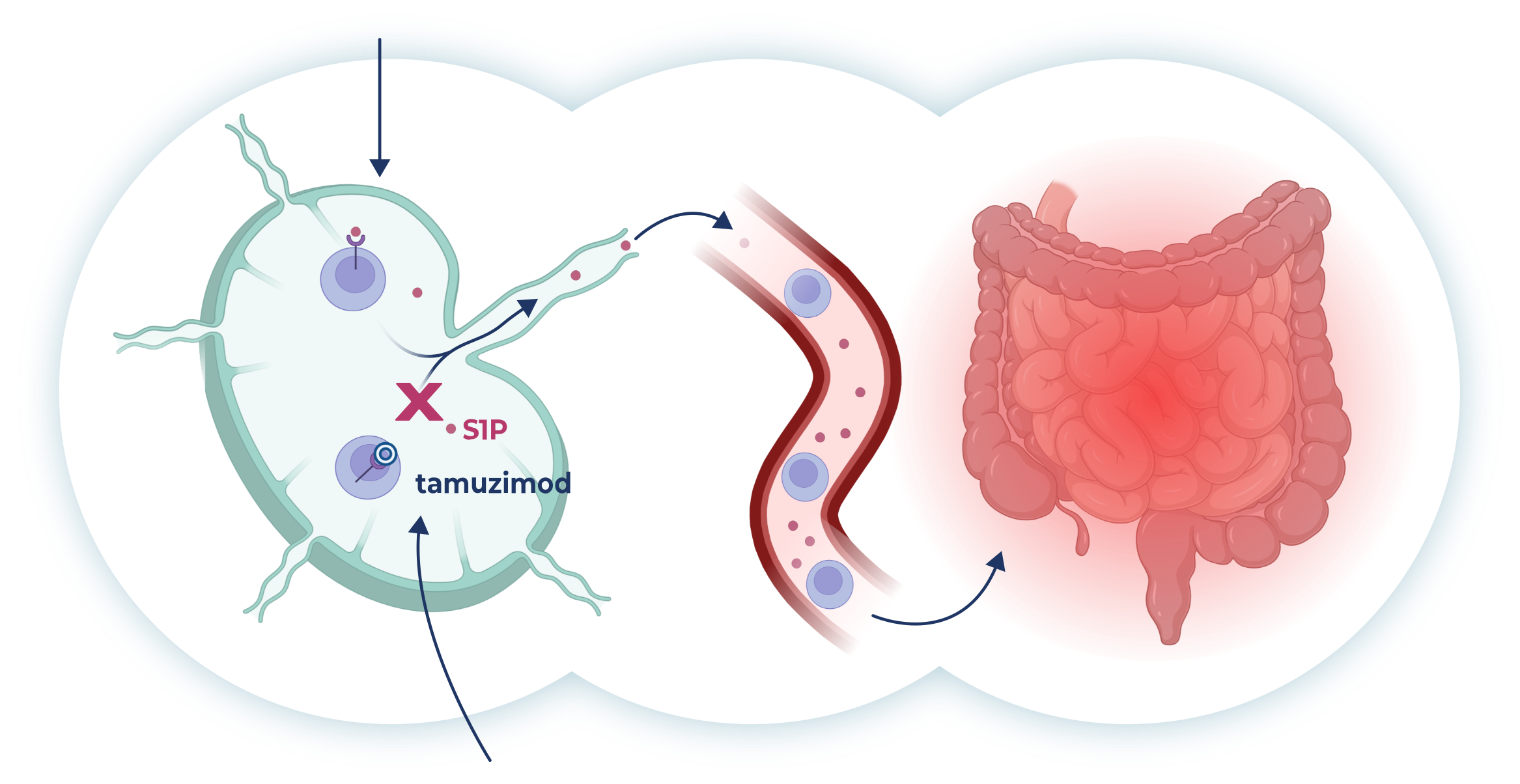
Tamuzimod is a S1P1 receptor modulator that binds to and internalizes the S1P1 receptor, preventing lymphocytes from recognizing this signal, thereby sequestering them in the lymph nodes with concomitant reduction in circulating lymphocytes. This disrupts the chronic inflammatory cycle and facilitates tissue healing and symptom resolution in diseases such as ulcerative colitis.
Ulcerative colitis
Tyrosine kinase 2 (TYK2) is a member of the Janus kinase (JAK) family of enzymes and plays a critical role in both innate and adaptive immunity by mediating type I interferon (IFNα) and the IL-12 and IL-23 signaling pathways.1 Overactive production of these cytokines is typical in patients with autoimmune disease and is a driver of disease pathology in conditions such as psoriasis, psoriatic arthritis, and IBD, among others. The essential role of TYK2 signaling in these diseases has been shown in studies of naturally occurring genetic mutations in humans and in genetic deletion/knockout animal disease models. Since JAK family enzymes regulate many important biological processes, selectively inhibiting TYK2 without inhibition of other JAK family enzymes could provide an optimal balance between reducing inflammation and preserving immune protection, offering a superior combination of efficacy and safety to existing therapies.
1. Gonciarz et al, Immunotherapy, 2021, 13(13), 1135; Rusinol et al, Int J Mol Sci, 2023, 24(4), 3391